FAQS
Get answers to some of the most common questions that ANA has received about COVID-19, including clinical care, PPE, mental health, and licensure.
What nurses need to know
- Clinical Information about COVID-19
- Post-Acute Sequelae of COVID-19 (PASC), Long COVID and Post-COVID Conditions
- Vaccine Information
- Keep yourself safe (COVID-19 info, PPE, etc.)
- Ethical Considerations
- Mental Health and Well-being
- Licensure and Credentialing guidelines
- Legislative and Regulatory
- Consumers (what to tell consumers/your patients)
Get involved
Clinical Information about COVID-19
Q: What is COVID-19?
A: COVID-19, also referred to as SARS-CoV2, is a novel coronavirus identified in the Hubei Province of Wuhan, China in December 2019. In the naming convention, the CO stands for corona, VI for virus and D for disease. Coronaviruses are not a new family of viruses. In humans, there are multiple strains that can cause mild respiratory symptoms or even the common cold. What is currently understood about COVID-19 is that it spreads person-to-person among close contacts via respiratory droplets produced from coughs or sneezes or droplets exhaled during talking. Transmission through the air (aerosol) can occur in specific settings, particularly in indoor, crowded and inadequately ventilated spaces, where infected person(s) spend long periods of time with others, such as restaurants, choir practices, fitness classes, nightclubs, offices and/or places of worship. It is also possible to spread COVID-19 via touching infected surfaces and then touching your nose, mouth, or eyes. Continued disease tracking and research has confirmed asymptomatic and pre-symptomatic spread of COVID-19 amongst the general population. According to the Center for Disease and Prevention (CDC) and World Health Organization (WHO), COVID-19 has an incubation period that ranges from 1-14 days, with symptoms appearing on average around 5-6 days following exposure. Symptoms associated with COVID-19 include mild to severe respiratory illness with symptoms of fever, cough, and shortness of breath; loss of taste/smell; headache; muscle and joint aches; nausea and vomiting, and diarrhea.
Q: What are COVID-19 Variants?
A: According to the CDC, viruses constantly change through mutation and sometimes these mutations result in a new variant of the virus. Some variants emerge and disappear while others persist. New variants of the virus are expected to occur. Taking steps to reduce the spread of infection, including getting an updated COVID-19 vaccination, wearing a mask in public indoor settings in areas of substantial or high levels of COVID-19 hospitalizations, and utilizing Covid-19 testing are the best way to slow the emergence of new variants.
Q: How is COVID-19 transmitted?
A: COVID-19 is primarily transmitted from respiratory droplets sprayed during a cough or sneeze or exhaled when someone talks or breathes. These droplets land in the mouth or nose of those within close contact of an infected individual and can land on surfaces nearby. The size of respiratory droplets can vary from large to small. Larger droplets travel a shorter distance and land within the mouth or nose of a close contact or on a surface nearby. Smaller droplets have the potential to travel further or remain suspended in the air for minutes to hours.
Aerosol transmission can also occur in specific settings, particularly in indoor, crowded and inadequately ventilated spaces, where infected person(s) spend long periods of time with others, such as restaurants, choir practices, fitness classes, nightclubs, offices and/or places of worship. More studies are underway to better understand the conditions in which aerosol transmission is occurring.
There are procedures that can aerosolize the virus resulting in airborne transmission of the virus. These procedures include, but are not limited to positive pressure ventilation, endotracheal intubation or extubation, bronchoscopy, airway suction, ventilator care, tracheostomy care, Chest PT, nebulizer treatment, and sputum induction.
Q: Is there asymptomatic and pre-symptomatic transmission?
A: Asymptomatic and pre-symptomatic transmission of COVID-19 or SARS-CoV2 is confirmed. In fact, individuals can transmit the virus without ever experiencing symptoms or during the pre-symptomatic phase up to 48 hours before showing symptoms. The typical incubation cycle of SARS-CoV2 is 1-14 days with the average onset of symptoms by day 5-6. Most individuals (80%) will experience mild symptoms requiring home management of symptoms. Virus mutations have resulted in variants that are highly transmissible with a shorter incubation period. In fact, recent epidemiological data on cases and spread confirm virus transmission of the Delta variant by fully vaccinated people.
Q: Is there a treatment?
A: There are multiple treatment options available for mild to moderate COVID-19, and for those who test positive for COVID-19 who are at high risk of developing severe disease. Remember to test soon and treat early! If you test positive for COVID-19 and have one or more health conditions that increase your risk of becoming very sick, treatment may be available. Contact a health professional right away after a positive test to determine if you may be eligible, even if your symptoms are mild right now. Depending on where you live, there may be a COVID-19 Test-To-Treat location near you. Don’t delay, treatment must be started within the first few days to be effective.
Here are links where you can learn more about the current available treatment options (at-home and in-hospital):
• National Institutes of Health (NIH) COVID-19 Treatment Guidelines website
• U.S. Department of Health and Human Services (HHS) Possible Treatment Options for COVID-19 website
• Center for Disease Control and Prevention (CDC) Clinical Care Quick Reference for COVID-19 website
• U.S. Health & Human Services (HHS) COVID-19 Public Therapeutic Locator website
Q: What is an Emergency Use Authorization (EUA)?
A: During times of public health emergency or infectious disease outbreak when there is no adequate, approved, or an available alternative for the diagnosis, prevention, or treatment for a disease or condition, The US Food and Drug Administration may issue an EUA. An EUA is issued when available scientific evidence suggests reasonable believe that medical products, such as drugs, tests, and medical devices, may be effective in diagnosing or preventing serious or life-threatening diseases or conditions caused by the outbreak and the known and potential benefits of the product outweigh the know and potential risks of the product when utilized.
To learn more information about EUAs and how the FDA utilizes this tool to help make important medical products available during the COVID-19 pandemic watch the video What is an EUA?
Q: When do I need to isolate?
A: For the General Public:
Have you tested positive for COVID-19 or have mild symptoms and are waiting for test results?
Regardless of vaccination status, you should isolate from others when you have COVID-19. You should also isolate if you are sick and suspect that you have COVID-19 but do not yet have test results. If your results are positive, follow the full isolation recommendations below. If your results are negative, you can end your isolation.
If you test positive for COVID-19, stay home for at least 5 days and isolate from others in your home. You are likely most infectious during these first 5 days.
• Wear a high-quality mask if you must be around others at home and in public.
• Do not go places where you are unable to wear a mask.
• Do not travel.
• Stay home and separate from others as much as possible.
• Use a separate bathroom, if possible.
• Take steps to improve ventilation at home, if possible.
• Don’t share personal household items, like cups, towels, and utensils.
• Monitor your symptoms. If you have an emergency warning sign (like trouble breathing), seek emergency medical care immediately.
End isolation based on how serious your COVID-19 symptoms were.
If you had no symptoms, you may end isolation after day 5. If you had symptoms you may end isolation after day 5 if:
• You are fever-free for 24 hours (without the use of fever-reducing medication)
• Your symptoms are improving
If you still have fever or your other symptoms have not improved, continue to isolate until they improve.
- If you had moderate illness (if you experienced shortness of breath or had difficulty breathing), due to COVID-19, or you have a weakened immune system, you need to isolate through day 10.
- If you had severe illness (you were hospitalized), consult your doctor before ending isolation. Ending isolation without a viral test may not be an option for you.
- If you are unsure if your symptoms are moderate or severe, or if you have a weakened immune system, talk to a healthcare provider for further guidance.
After you have ended isolation, when you are feeling better (no fever without the use of fever-reducing medications and symptoms improving), wear your mask through day 10, OR, If you have access to antigen tests, you should consider using them. With two sequential negative tests 48 hours apart, you may remove your mask sooner than day 10.
After you have ended isolation, if your COVID-19 symptoms recur or worsen, restart your isolation. Talk to a healthcare provider if you have questions about your symptoms or when to end isolation.
Have you been exposed to someone with COVID-19?
If you were exposed to the virus that causes COVID-19 or have been told by a healthcare provider or public health authority that you were exposed, here are the steps that you should take, regardless of your vaccination status or if you have had a previous infection:
• Wear a mask as soon as you find out you were exposed.
• Take Precautions - wear a high-quality mask or respirator (e.g., N95) any time you are around others inside your home or indoors in public.
• Take extra precautions if you will be around people who are more likely to get very sick from COVID-19
• Do not go places where you are unable to wear a mask, including travel and public transportation settings.
• Watch for symptoms - fever (100.4°F or greater), cough, shortness of breath, other COVID-19 symptoms.
If you develop symptoms
• isolate immediately
• get tested
• stay home until you know the result
• If your test result is positive, follow the isolation recommendations.
Get tested at least 5 full days after your last exposure, test even if you don’t develop symptoms
• If you test Negative continue taking precautions through day 10. Wear a high-quality mask when around others at home and indoors in public
• If you test Positive Isolate immediately
A: For Healthcare Workers – On Dec. 23rd, 2021, the CDC issued their Interim Guidance for Managing Healthcare Personnel with SARS-CoV-2 Infection or Exposure to SARS-CoV-2. It states that in general, asymptomatic healthcare providers (HCP) who have had a higher-risk exposure do not require work restriction if they have received all COVID-19 vaccine doses, including booster dose, as recommended by the CDC and do not develop symptoms or test positive for SARS-CoV-2.
Return to Work Criteria for HCP with confirmed SARS-CoV-2 Infection varies depending on the severity of the illness, the immune status of the HCP, and the strategies being used to mitigate staffing shortages at the facility in which they work. A detailed description of the current recommendations can be found here.
For more information on the ANA response to these new recommendations, read our December 29, 2021 statement, ANA Deeply Concerned about CDC’s Updated Guidance, Urges Policymakers to Prioritize Health Care Workers’ Safety as Surge Continues.
Q: When should I return to work after I have been sick with COVID-19?
A: Prior to your return to work, discuss with your nurse manager and infection control / employee health team which process and guidelines to follow. The CDC has outlined and updated the return to work criteria following COVID-19 exposure and recovery These criteria are based upon symptom resolution/recovery, test results, and time of exposure.
Read our December 29, 2021 statement, ANA Deeply Concerned about CDC’s Updated Guidance, Urges Policymakers to Prioritize Health Care Workers’ Safety as Surge Continues.
Q: I have been infected. Can I get COVID-19 again?
A: Cases of reinfection with Covid-19 have been reported. Reinfection means a person was infected, recovered, and then later became infected again. After recovering from COVID-19, most individuals will have some protection from repeat infections. However, reinfections do occur after COVID-19. Scientists are working to understand our immune response to COVID-19 and the scientific evidence behind exposure and implications for re-infection. At this time, whether you have had COVID-19 in the past or not, the best way to prevent infection is to take steps to protect yourself.
Q: If a patient tests positive for the flu, should they still be tested for COVID-19?
A: Yes, the flu and COVID-19 are two different viruses. Presence of the flu does not rule out co-occurrence of COVID-19. Some of the symptoms of flu and COVID-19 are similar, making it hard to tell the difference between them based on symptoms alone. Diagnostic testing can help determine if you are sick with flu or COVID-19.
Q: I have questions about Covid-19 testing. Where can I get more information?
A: Remember to test soon and treat early! If you test positive for COVID-19 and have one or more health conditions that increase your risk of becoming very sick, treatment may be available. Contact a health professional right away after a positive test to determine if you may be eligible, even if your symptoms are mild right now. Depending on where you live, there may be a COVID-19 Test-To-Treat location near you. Don’t delay, treatment must be started within the first few days to be effective. To find general information on when to do a Covid-19 test, understand testing options and find healthcare worker testing guidance, visit the CDC Covid-19 Testing webpage.
Q: What about immunity? Is there an antibody test?
A: An antibody test or serological test measures the number of antibodies in the blood developed against a virus. These tests are important in identifying who has successfully overcome the virus and developed an immune response, however the immune response, including duration of immunity, is not yet fully understood. There are multiple antibody tests currently approved by the FDA including the first standalone at-home sample collection kit. Learn more here. Given the number of test kits currently available for use, the FDA requires authorization of each kit to remain on the market. For a comprehensive and updated list of current EUAs available for serologic and diagnostic tests, visit the FDA’s EUA page.
Additional studies on serological tests are underway as researchers continue to develop and refine methods to expand the identification of those exposed to COVID-19.
Q: Are children carriers of COVID-19?
A: It is unclear whether children are as susceptible to infection by Covid-19 compared with adults, and whether they can transmit the virus as effectively as adults. Recent evidence suggests that children likely have the same or higher viral loads in their nasopharynx compared with adults and that children can spread the virus effectively in households and camp settings.
The emergence of variants such as the Omicron variant have posed a risk to children, particularly among children who remain ineligible for vaccination. Children can still acquire COVID-19 and must adhere to the same precautions – mask wearing, hand hygiene, social distancing, avoidance of touching the face, nose, mouth, and eyes–to minimize their risk for exposure to COVID-19. Find more information for care of pediatric patients here. As we continue to learn about COVID-19, epidemiological data has revealed a Multisystem Inflammatory Syndrome (MIS-C) in children associated with COVID-19. MIS-C is presenting is previously healthy children and is characterized by a severe inflammatory syndrome with Kawasaki disease-like features. Published May 14, 2020 via the CDC Health Alert Network is a case definition for MIS-C.
Q: Are smokers at greater risk?
A: Studies indicate that people who smoke are more likely to have severe symptoms from COVID-19 compared to those who did not smoke. This may be in part due to damage caused by smoking and vaping to the airway and inflammation within the respiratory system that affects the immune system. To help your patients quit smoking, visit our cessation resource page or refer them to 1-800-QUIT-NOW for tips for quitting smoking cigarettes and vaping.
Q: What about the homeless population and self-quarantine and isolation?
A: Lack of housing and shelter increases the risk of poor health outcomes resulting from COVID-19 given the lack of access to health care, facilities for personal hygiene and care, and protection from environmental elements. The CDC has outlined Guidance on Management of COVID-19 in Homeless Service Sites and in Correctional and Detention Facilities.
Q: I’m a psychiatric nurse, how do I continue to provide care?
A: Continuation of psychiatric care and support is vital during these unprecedented times. The American Psychiatric Association has available guidance released by the Department of Health and Human Services, FDA, and at the state level related to COVID-19 to assist with providing mental health and substance use services.
Additional COVID-19 resources can be found through the American Psychiatric Nurses Association
Q: How do I protect my patients in a primary care setting?
A:
- Set up a telehealth service for patients to utilize to minimize in-person office visits. Rotate clinicians through the telemedicine and on-call portal for on-going access during office hours.
- For in-person appointments, designate staff to call ahead of the appointment to screen for exposure and symptoms and for instructions prior to arrival – how to maintain physical distance, provide paperwork to complete at home in advance of the visit, request insurance information for prior authorization and verification to minimize time waiting in the office.
- Instruct patients visiting the office to wear a well-fitting mask over their mouth and nose. Experts recommend everyone upgrade their mask to a high filtration respirator for optimal protection. Use this resource to promote the highest level of mask protection in your community. This resource is also available in Spanish.
- Instruct patients to avoid touching the face, eyes, nose, or mouth.
- Encourage physical distancing in common areas by separating chairs and outlining designated areas to stand that are six feet apart.
- Disinfect pens and clipboards after every use.
- Provide hand sanitizer in common areas, at the door upon arrival and exit, in all exam rooms, and post clear signage educating patients on proper cough and sneeze etiquette and hand hygiene.
- Arrange prescriptions for a 90-day supply to minimize office returns for orders for refills.
Additional information from the CDC can be found here.
Q: How do I set up a telehealth program for my patients?
A: The practice of telehealth will provide a continuation of health services while protecting providers and patients from exposure to COVID-19. To successfully implement a telehealth program, it is essential to understand laws and regulations associated with privacy protection and virtual care. Learn more about starting a telehealth program through the American Association of Nurse Practitioners (AANP) Telehealth Updates.
Other useful resources include The U.S. Department of Health and Human Services FAQs on Telehealth and HIPAA during the COVID-19 nationwide public health emergency.
Q: My patient is positive, but not sick enough to be admitted. What instructions do I provide for home care?
A: If you have a patient with COVID-19 who is returning home, the CDC provides the following guidance to help prevent COVID-19 from spreading between people in homes and communities.
- Remember to test soon and treat early! If you test positive for COVID-19 and have one or more health conditions that increase your risk of becoming very sick, treatment may be available.
- STAY HOME except to get medical care, do not use public transportation or taxis if sick.
- Call first before visiting your health care provider. Notify them of your symptoms and the need for evaluation for COVID-19. Follow the instructions provided by your health care team.
- Separate yourself from other people in your home, isolate yourself to a single room, utilize a separate bathroom.
- Wear a facemask as instructed if you are sick. Family members providing care should wear a facemask when in the same room, even those family members who have received the COVID-19 vaccine. Experts recommend everyone upgrade their mask to a high filtration respirator for optimal protection. Use this resource to promote the highest level of mask protection.
- Use your elbow to cover your coughs and sneezes or cough and sneeze in a tissue and dispose in a lined trash can. Wash your hands with soap and water afterwards.
- Wash your hands frequently with soap and water for at least 20 seconds.
- Avoid sharing household items such as eating utensils, and clean sheets with hot water and detergent.
- Surfaces within the home must be disinfected frequently.
- Monitor your symptoms, follow instructions for when to seek emergency medical care if warranted.
Post-Acute Sequelae of COVID-19 (PASC), Long COVID and Post-COVID Conditions
Q: What are Post-Acute Sequelae of COVID-19 (PASC), Long COVID and Post-COVID Conditions?
A: Post-acute sequelae of COVID-19 (PASC), commonly referred to as Long COVID, is a condition marked by persistent COVID-19 symptoms or the onset of new symptoms following recovery from acute COVID-19. PASC is characterized by new, continuing, or recurring respiratory, neurological, psychological, and cardiac problems occurring four or more weeks after initial COVID-19 infection. PASC can affect anyone including those who had asymptomatic and mild COVID-19 infection. The causes of PASC remain unknown and the research is ongoing to further understand PASC. If you or a family member is experiencing prolonged symptoms associated with COVID-19, we encourage you to contact a medical clinician and seek a medical evaluation. Here is additional information:
- CDC webpage on Long COVID or Post-COVID Conditions
- Yale Medicine - Long COVID (Post-Acute Sequelae of SARS CoV-2 infection, PASC) Fact Sheet
- Johns Hopkins Medicine webpage on COVID ‘Long Haulers’: Long-Term Effects of COVID-19
Q: Is there a test for Post-Acute Sequelae of COVID-19 (PASC), Long Covid and Post-COVID Conditions?
A: No, according to the CDC there is no single test for post-COVID conditions. While most people with post-COVID conditions have evidence of infection or COVID-19 illness, in some cases, a person with post-COVID conditions may not have tested positive for the virus or known they were infected.
Q: Is there a treatment for Post-Acute Sequelae of COVID-19 (PASC), or Long Covid?
A: According to the CDC, having a post-COVID condition or supporting someone with a post-COVID condition can be challenging. It can be difficult to care for yourself or loved ones, especially when there are few or no immediate answers or solutions. However, there are ways to help relieve some of the additional burdens of experiencing or caring for someone with a new and unknown condition. Here is additional information:
Q: Is Post-Acute Sequelae of COVID-19 (PASC), or Long Covid Considered a disability by the ADA?
A: Yes, according to the Office for Civil Rights of the Department of Health and Human Services, and the Civil Rights Division of the Department of Justice, Long COVID can be a disability under the ADA, Section 504, and Section 1557 if it substantially limits one or more major life activities. Here is additional information:
- HHS Guidance on “Long COVID” as a Disability Under the ADA, Section 504, and Section 1557
- U.S. Department of Labor Office of Disability Employment Policy - COVID-19 and Long COVID-19
Q: What is the U.S. Government doing about Post-Acute Sequelae of COVID-19 (PASC), or Long Covid?
A: On April 5th, 2022 the White House issued a Presidential Memorandum directing the Secretary of Health and Human Services (HHS) to coordinate a new interagency national research action plan on Long COVID. “The effort will advance progress in prevention, diagnosis, treatment, and provision of services, supports, and interventions for individuals experiencing Long COVID and associated conditions”, and it “directs HHS to issue a report outlining services and supports across federal agencies to assist people experiencing Long COVID, individuals who are dealing with a COVID-related loss, and people who are experiencing mental health and substance use issues related to the pandemic. “
Q: Are there Research studies on the long-term effects of COVID, and how can I participate?
A: The National Institutes of Health (NIH) created the RECOVER Initiative to understand, prevent, and treat long-term health effects related to COVID. Here you can find information, share your experience, and volunteer to participate in research:
- MMWR Post–COVID Conditions Among Adult COVID-19 Survivors Aged 18–64 and ≥65 Years — United States, March 2020–November 2021
- NIH RECOVER: Researching COVID to Enhance Recovery
- NIH Find a RECOVER study site and join today
Vaccine Information
The CDC recommends everyone stay up to date with COVID-19 vaccination:
• The CDC recommends everyone eligible get the 2023–2024 updated COVID-19 vaccines.
• Everyone aged 5 years and older should get 1 dose of the updated Pfizer-BioNTech or Moderna COVID-19 vaccine to protect against serious illness from COVID-19. Adults ages 65 years and over should receive an additional updated 2023-2024 COVID-19 vaccine dose.
• People who are moderately or severely immunocompromised may get additional doses of updated COVID-19 vaccine.
• Children aged 6 months–4 years need multiple doses of COVID-19 vaccines to be up to date, including at least 1 dose of updated COVID-19 vaccine.
Go to vaccines.gov to locate a COVID-19 vaccination site near you
Q: What are the Updated Covid-19 vaccines?
A: The Vaccines authorized by the U.S. Food and Drug Administration (FDA) currently include:
• Pfizer-BioNTech and Moderna COVID-19 vaccines which are mRNA vaccines.
• Novavax COVID-19 vaccine which is a protein subunit vaccine.
• J&J/Janssen COVID-19 vaccine, a viral vector vaccine has expired and is no longer available for use in the United States as of May 6, 2023.
• For more information, see the CDC Overview of COVID-19 Vaccines.
Find information on COVID-19 Vaccines for Moderately or Severely Immunocompromised People here.
Visit the ANA Covid-19 Vaccine Resources webpage, and the CDC Coronavirus Disease 2019 (COVID-19) Vaccines webpage for additional information. Healthcare clinicians can find additional information on the CDC Interim Clinical Considerations webpage. See also the CDC Frequently Asked Questions about COVID-19 Vaccination webpage. To learn more about the EUA process, click here.
Vaccine Information for Children
Q: Can children and teens be vaccinated against Covid-19?
A: Yes, children 6 months and older can be vaccinated against COVID-19. For the best protection, the CDC recommends COVID-19 vaccines for everyone 6 months and older.
Q: Where can I find a pediatric vaccination site?
A: Go to vaccines.gov to locate a COVID-19 vaccination site near you.
Q: What is the difference between a pediatric and adult COVID-19 Vaccine dose?
A: The COVID-19 vaccines for children have the same active ingredients as the vaccines given to adults. However, children receive a smaller, age-appropriate dose that is the right size for them. Find answers to frequently asked questions on COVID-19 vaccines for children here.
Q: Can I draw up a COVID-19 Vaccine pediatric dose from an adult formulation?
A: No. There are differences in the inactive ingredients in the adult and pediatric vaccines. Pediatric doses must be drawn from a designated pediatric vial.
- Find dosage information for Pfizer-BioNTech COVID-19 Vaccines here.
- Find dosage information for Moderna COVID-19 Vaccines here.
Q: What are the side effects following vaccination for pediatric patients?
A: Common side effects include injection site pain, redness or swelling. Other common side effects include fatigue, headache, fever, chills, and muscle aches.
Q: Can a pediatric patient receive an influenza and COVID-19 vaccine during the same visit?
A: Yes. Co-administration of influenza and COVID-19 vaccines is not contraindicated in the pediatric population. Find additional information here.
Q: Where do I report pediatric adverse reactions?
A: Adverse events are reported and tracked via the Vaccine Adverse Event Reporting System (VAERS). Parents are also encouraged to monitor and report their child’s response to vaccination using V-Safe a smart phone based vaccine response reporting app.
Additional Resources:
- CDC 6 Things to Know about COVID-19 Vaccination for Children: https://www.cdc.gov/vaccines/covid-19/planning/children/6-things-to-know.html
- CDC COVID-19 Vaccination Program Provider Agreement: https://scdhec.gov/sites/default/files/media/document/COVID19-Vaccination_Program_Provider_Agreement_and_Profile_Form.pdf
- Vaccine Adverse Event Reporting System: https://vaers.hhs.gov/index.html
- Vaccine Safety Datalink: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html
- Clinical Immunization Safety Assessment Project: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/
- VAERS Table of Reportable Events Following Vaccination: https://vaers.hhs.gov/docs/VAERS_Table_of_Reportable_Events_Following_Vaccination.pdf
Vaccine General Information
Q: How do you become a provider of COVID-19 vaccines?
A: COVID-19 vaccines are administered by healthcare professionals enrolled as vaccination providers through a health practice or organizations. Contact your health system administrator about your health systems enrollment eligibility. You can also visit the CDC’s COVID-19 vaccine provider enrollment page for eligibility and enrollment criteria at: https://www.cdc.gov/vaccines/covid-19/provider-enrollment.html
You can also volunteer through your local Medical Reserve Corps to support vaccination programs in your community.
Q: How do I educate my patients?
A: Ask about vaccination status during health care visits and inform of eligibility of updated doses if indicated by their medical profile or known exposure risk category.
Have educational materials in multiple languages to appropriately educate all patients.
If working in an office or a community administration site, you can use your secure messaging services such as email or text to notify patients of the availability of updated COVID-19 doses. For patients who do not use email or text messaging, have staff call patients.
Remember to also remind patients that vaccines are safe and effective.
Q: Is it safe to administer flu shots and COVID-19 vaccines at the same visit?
A: Influenza season has started. Flu shots help prevent the spread of the flu. It is safe to administer the flu shot and COVID-19 vaccine during the same visit. If co-administering the influenza and COVID-19 vaccine, administer each injection in a different injection site.
For more information about Co-administration of influenza and COVID-19 vaccines, watch this video from the CDC here.
For more information about COVID-19 vaccines, visit ANA’s COVID-19 Resource Center.
Q: *What are the differences in technology development between the vaccine candidates?
A: There are several platforms being used to develop the COVID-19 vaccine candidates. They are briefly explained in the table below.
| Type | Description | Example |
| mRNA | Fragments are inserted into human cells to reprogram them to produce pathogen antigens, which stimulate an immune response against the pathogen. How it works: https://www.youtube.com/watch?v=w4sUuFBEo2g |
Pfizer / BioNTech Moderna |
| Viral Vector | Nonreplicating Vector – Based on recombinant viral vectors that are sufficient to induce host immune responses but cannot replicate inside host cells. How it works: https://www.gavi.org/vaccineswork/what-are-viral-vector-based-vaccines-and-how-could-they-be-used-against-covid-19 |
Johnson&Johnson/Janssen candidate Oxford/AstraZeneca candidate |
| Protein-Based | Vaccine made from small proteins or peptides that contain epitopes that are then presented to and recognized by T-cells as target tumor antigen and generate an immune response. How it works: https://www.gavi.org/vaccineswork/what-are-protein-subunit-vaccines-and-how-could-they-be-used-against-covid-19 |
Novavax candidate |
Additional Resources:
- ASHP Vaccine Candidate Tracking Table: https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/Vaccine-candidate-tracking-table.ashx
- CDC Understanding and Explaining mRNA COVID-19 Vaccines: https://www.cdc.gov/vaccines/covid-19/hcp/mrna-vaccine-basics.html
Q: *What are the various regulatory review pathways that the FDA can consider in order to release a vaccine (BLA, EUA, Expanded access)?
A: The regulatory approval pathways are summarized below. The FDA will rely on the Vaccines and Related Biological Products Advisory Committee (VRBPAC) to make recommendations based on its evaluation of safety, efficacy, and appropriate use data available for the COVID-19 vaccine candidates.
Biologics License Application (BLA):
Once preclinical and clinical development programs have been successfully completed, companies submit a BLA to the FDA. A BLA is a comprehensive submission which includes preclinical and clinical data and information, as well as details of the manufacturing process and facility(ies). The BLA is the official request for permission to introduce a biologic product, including a vaccine, into interstate commerce.
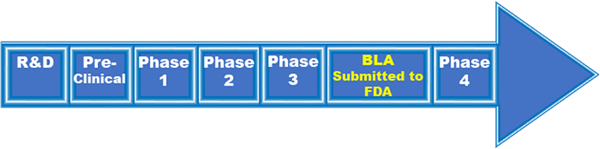
Emergency Use Authorization (EUA):
During a public health emergency, if certain criteria are met, manufacturers may submit a request for EUA to FDA to facilitate the availability and use of their vaccine during this time.
Under an EUA, the Commissioner may allow unapproved drug, unlicensed vaccine, or uncleared device to be used in a public health emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused when there are no adequate, approved, and available alternatives.
The EUA process is different than an FDA approval or clearance. With each EUA decision, FDA weighs known and potential benefits of product against known and potential risks.
- EUAs helped speed access to COVID-19 diagnostic tests, N95 respirators, and remdesivir.
- COVID-19 vaccines: FDA prefers phase-3 studies be completed. EUA sooner could impair efficacy + safety determination.
Expanded access:
This allows investigational drugs, biologics, or medical devices that have not yet been approved or cleared by FDA to be used for a patient with an immediately life-threatening condition or serious disease for treatment outside of clinical trials when no comparable or satisfactory alternative therapy options are available.
Currently, expanded access is one pathway for use of COVID-19 convalescent plasma for patients with serious or immediately life-threatening COVID-19 disease who are not eligible for or who are unable to participate in randomized clinical trials.
Additional Resources:
- Understanding the Regulatory Terminology of Potential Preventions and Treatments for COVID-19
- FDA BLA Resources
- FDA EUA Guidance: https://www.fda.gov/media/97321/download
- FDA Vaccines and Related Biological Products Advisory Committee https://www.fda.gov/advisory-committees/blood-vaccines-and-other-biologics/vaccines-and-related-biological-products-advisory-committee
Q: *What are immediate side effects to expect from the COVID-19 vaccine?
A: As your body gets used to the COVID vaccine in your system it is common to have minor effects. Reported symptoms include pain at the injection site, fever, fatigue, and headaches and are worse after the second dose. Other symptoms can include muscle and joint pain, cough, feeling ‘hungover’, and shortness of breath.
Q: Is it safe for me to get a COVID-19 vaccine if I am pregnant or planning to get pregnant in the future?
A: Yes. People who are pregnant, or who want to get pregnant in the future may receive the COVID-19 vaccine. Based on current knowledge, experts believe that COVID-19 vaccines are unlikely to pose a risk to a person who is pregnant or trying to become pregnant in the short or long term.
Additional Resources
- Association of Women’s Health, Obstetric and Neonatal Nurses Covid-19 Practice Guidance https://www.awhonn.org/novel-coronavirus-covid-19/covid19-practice-guidance/
- CDC Facts about the COVID-19 Vaccines https://www.cdc.gov/coronavirus/2019-ncov/vaccines/facts.html
- CDC Vaccination Considerations for People who are Pregnant or Breastfeeding https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
- CDC Vaccines for People Who Would Like to Have a Baby https://www.cdc.gov/coronavirus/2019-ncov/vaccines/planning-for-pregnancy.html
- Vaccinating Pregnant and Lactating Patients Against COVID-19 https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19
Q: *What should I do if I or a patient experiences an adverse event from the vaccine?
A: Monitoring for safety signals of the new vaccines against SARS-CoV-2 after their approval and/or authorization is an important responsibility of the vaccinating workforce. In addition, it is an expectation outlined in the CDC COVID-19 Vaccination Program Provider Agreement. An “adverse event following immunization” is an adverse health problem or condition that happens after vaccination. To continuously monitor the safety of the new COVID-19 vaccines, the CDC will be leveraging existing vaccine safety monitoring infrastructure, including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink, and Clinical Immunization Safety Assessment Project. Healthcare organizations often have internal safety reporting systems as well. Healthcare providers are required by law to report to VAERS when any adverse event listed in the VAERS Table of Reportable Events Following Vaccination occurs within the specified timeframe and when an adverse event listed by the vaccine manufacturer as a contraindication to future doses of the vaccine occurs.
Additional Resources:
- CDC COVID-19 Vaccination Program Provider Agreement: https://scdhec.gov/sites/default/files/media/document/COVID19-Vaccination_Program_Provider_Agreement_and_Profile_Form.pdf
- Vaccine Adverse Event Reporting System: https://vaers.hhs.gov/index.html
- Vaccine Safety Datalink: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html
- Clinical Immunization Safety Assessment Project: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/
- VAERS Table of Reportable Events Following Vaccination: https://vaers.hhs.gov/docs/VAERS_Table_of_Reportable_Events_Following_Vaccination.pdf
Q: What are the storage and handling considerations for the various Covid-19 Vaccines?
A: The Pfizer/BioNTech vaccine (Comirnaty) information can be found at: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
The Moderna vaccine information can be found at: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html
The Novavax vaccine information can be found at: https://www.cdc.gov/vaccines/covid-19/info-by-product/novavax/storage.html
Additional Resources:
- CDC Vaccine Storage and Handling: https://www.cdc.gov/vaccines/hcp/admin/storage-handling.html
Q: How can I help stop the spread of COVID-19 vaccine misinformation?
A: As trusted healthcare professionals, patients will look to nurses for reliable information about the COVID-19 vaccines. Vaccine acceptance will increase in line with increased confidence in the vaccines, and the spread of misinformation on social media and through other channels can affect COVID-19 vaccine confidence. The first step to addressing misinformation about COVID-19 vaccines in your community is learning more about it, including where it starts and when, why, and how it is spreading and evolving. Strategies to address misinformation can be found at: https://www.cdc.gov/vaccines/covid-19/health-departments/addressing-vaccine-misinformation.html
Nurses are professionally accountable for the information they provide to the public. Any nurse who disseminates misleading or incorrect information pertaining to COVID-19, vaccines and associated treatment through verbal or written methods, including social media, not only jeopardizes the health and well-being of the public, but may place their license and career in jeopardy as well. Read the ANA Policy Statement: Dissemination of Non-scientific and Misleading COVID-19 Information by Nurses here.
Additional Resources:
- CDC Vaccinate with Confidence program: https://www.cdc.gov/vaccines/partners/vaccinate-with-confidence.html
- CDC Talking to Patients about COVID-19 Vaccines: https://www.cdc.gov/vaccines/covid-19/hcp/index.html
- When a Co-Worker Spreads Misinformation, American Nurse July 2021: https://myamericannurse.com/Digital/July_2021/#page=35
Please note – content marked with an * was developed in conjunction with the American Society of Health-System Pharmacists (ASHP).
About the American Society of Health-System Pharmacists (ASHP)
ASHP represents pharmacists who serve as patient care providers in acute and ambulatory settings. The organization’s over 55,000 members include pharmacists, student pharmacists, and pharmacy technicians. For more than 75 years, ASHP has been at the forefront of efforts to improve medication use and enhance patient safety. Visit ASHP online at www.ashp.org. Access the ASHP COVID-19 Resource Center at https://www.ashp.org/COVID-19.
Keep yourself safe
Q: How should I protect myself from this virus?
A: First and foremost, if you are medically able to receive a COVID-19 vaccine, an updated vaccination dose is strongly encouraged to prevent severe illness, hospitalization, or death from COVID-19 the disease caused the coronavirus SARS CoV-2. Fully vaccinated people can safely resume most activities they did prior to the pandemic, but mask use is still recommended in areas where there is high virus transmission. When making decisions about your individual prevention strategies and behaviors, consider the level of COVID hospitalizations where you live, work, or visit, and make decisions based on whether you or someone you have close contact with is at high risk for severe illness. If wearing a mask, experts recommend you upgrade your mask to a high filtration respirator if you want optimal protection. Continue to practice hand hygiene, and if you have symptoms of COVID-19 stay home if sick and seek testing for confirmation. Remember to test soon and treat early! If you test positive for COVID-19 and have one or more health conditions that increase your risk of becoming very sick, treatment may be available. Contact a health professional right away after a positive test to determine if you may be eligible, even if your symptoms are mild right now. Depending on where you live, there may be a COVID-19 Test-To-Treat location near you. Don’t delay, treatment must be started within the first few days to be effective. If you are immune compromised, live with, or closely interact with someone who is medically vulnerable or unvaccinated, wearing a mask, even if vaccinated, will help lower their risk of exposure. For those who are unvaccinated, or vaccinated, everyday measures to protect yourself from the virus include frequent hand hygiene, wearing a face mask in areas with high COVID-19 hospitalizations, avoiding contact with people who have suspected or confirmed COVID-19, improving indoor ventilation and spending more time outdoors, and most importantly, staying home if you are sick. If you are at higher risk for severe illness, additional information is found here.
See also the CDC How to Protect Yourself and Others webpage
If you are health care personnel:
- Adhere to the standards for donning and doffing PPE when caring for COVID-19 patients.
- Avoid touching your N95 respirator, facemask, eye goggles, and face shield if wearing during extended use.
- Wash your hands before donning all PPE. When doffing PPE, wash your hands before doffing your goggles, N95 respirator, and face shield, and again after all PPE is doffed.
- Wash your hands frequently with soap and warm water.
- Doff PPE before breaking for meals and taking trips to the rest room.
- Practice hand hygiene before and after going to the restroom and before eating.
- Eat meals in non-clinical areas.
- Disinfect your cell phone frequently, place your cellphone in a clear sealable bag that serves as a barrier, discard of the bag before going home, disinfect your cell phone before entering your home.
- Change your scrubs and shoes if possible before returning home.
- Find additional CDC infection prevention and control recommendations here.
- See also: Your COVID-19 Toolkit – find information on masks, treatments, vaccines, and testing in your community.
Q: Is fit testing for an N95 respirator still required?
A: Yes, fit testing ensures the N95 respirator forms a seal around the mouth and nose. If you have not been fit tested for an N95 respirator, your organization or infection control department must provide “just-in-time” fit testing to ensure the N95 respirator is worn properly. Contact your facility’s occupational health department or infection control personnel for your organization’s fit testing requirements.
On June 21, 2021, the U.S. Department of Labor's OSHA issued an Emergency Temporary Standard (ETS) to protect healthcare workers from contracting the Coronavirus Covid-19. This ETS established new requirements for settings where employees provide health care or health support services, including hospitals, skilled nursing homes and home health. On December 27, 2021, OSHA withdrew the non-recordkeeping portions of the healthcare ETS. The COVID-19 log and reporting provisions, 29 CFR 1910.502(q)(2)(ii), (q)(3)(ii)-(iv), and (r), remain in effect. To find out more, see the OSHA Covid-19 ETS Webpage.
Q: Can I reuse, or decontaminate my N95 Respirator?
A: The American Nurses Association continues to advocate aggressively for access to new unused PPE to ensure the ultimate protection of HCP when caring for COVID-19 patients. Read our full statement on the use of decontamination systems here.
As of May 27, 2021, The Food and Drug Administration (FDA) recommends health care personnel and facilities transition away from crisis capacity conservation strategies such as decontaminating disposable respirators for reuse, and the use of non-NIOSH approved disposable respirators such a KN95s. It is recommended that health care facilities increase inventory of available NIOSH-approved respirators including N95s, Elastomeric respirators, including elastomeric respirators without an exhalation valve that can be used in operating rooms, and Powered air-purifying respirators (PAPRs).
Q: What about the use of cloth masks?
A: Within the health care setting ANA maintains that all personnel have access to the highest level of respiratory protection to minimize exposure risk to COVID-19. This includes use of the N95 respirator, face shield, or goggles for eye protection, gown, and gloves. A cloth mask alone when providing care in a medical setting does not provide sufficient respiratory protection from COVID-19.
Outside of the health care setting, fully vaccinated individuals can go without a mask if permitted to do so by their state, locale, or territory. However, it is recommended that you continue to wear your mask even if vaccinated in areas with high transmission. A mask will maximize your protection and prevent spreading it to others. if you are wearing a mask, experts recommend you upgrade your mask to a high filtration respirator if you want optimal protection.
When making decisions about community and individual prevention strategies and behaviors, health officials and others should consider the level of COVID-19 hospitalizations in the area where they live, work, or visit, and individuals should make decisions based on whether they, or their close contacts are at high risk for severe illness.
Regardless of the level of transmission in your area, if you have a weakened immune system, are at increased risk for severe disease, wearing a mask provides additional protection. In general, the more closely you interact with others and the longer that interaction, the higher the risk of COVID-19 spread, and remember, continue to wear a mask where required by laws, rules, regulations, or local guidance.
Q: What should I do to keep my family safe when I go home after my shift?
A: Vaccination is the best way to minimize your COVID-19 risk. COVID-19 is primarily spread through respiratory droplets from coughs and sneezes and droplets exhaled when talking. Transmission through the air (aerosol) can occur in specific settings, particularly in indoor, crowded and inadequately ventilated spaces, where infected person(s) spend long periods of time with others. The virus can also be contracted by touching an infected surface and then touching the face, eyes, nose, or mouth. Therefore, cleaning hands and wiping down frequently touched objects, such as a cell phone or tablet, that are commonly transported between home and work is an important step to take prior to returning home. The U.S. Environmental Protection Agency (EPA) has developed a list of products suitable for use against COVID-19.
Additional tips include:
- Remove scrubs and shoes worn while providing care before entry into the home. If possible, wear hospital provided scrubs or carry a change of clothes to work including shoes.
- Remove shoes before entry into the home.
- Keep scrubs contained in a disposable bag, wash in hot water with detergent separately from other laundry items. Dispose of the bag in a lined trashcan once emptied into the washer. Wash your hands.
- Disinfect your cellphone frequently while on the unit and again just before leaving. Consider storing your cellphone in a clear sealable bag as a barrier.
- Leave high touch objects that are not essential for home on your unit, such as pens and clipboards used during your shift, disinfect frequently during the course of your shift.
- If using a personal stethoscope, leave on the unit in a locker, and disinfect before and after use with every patient.
- Wash hands with soap and water upon leaving the unit and again upon entry into the home.
Guidelines have changed during the course of the pandemic. Encourage your family members to get vaccinated and boosted. When making decisions about individual prevention strategies and behaviors, you should consider the level of COVID-19 hospitalizations in the area where you live, work, or visit, and make decisions based on whether an individual, or their close contacts are at high risk for severe illness.
See also the CDC How to Protect Yourself and Others webpage.
Q: Can I ask for reassignment if I am within a vulnerable population?
A: Individuals over the age of 65 and with chronic underlying medical conditions are at increased risk of severe morbidity and mortality as a result of COVID-19. If you fall within this category, have a conversation with your employer about redeployment to support telehealth services, transfer to units without COVID-19 patients, or assignment to non-COVID-19 patients needing care. It is also crucial to have access to the appropriate PPE to minimize the risk of exposure.
Q: I’ve been re-deployed to new unit and I don't feel comfortable with the assignment. What should I do?
A: As we look at the re-deployment of nurses to meet patient surge in response to the pandemic, staffing to meet demands does not replace the need to give consideration to:
- Assessment of the skill of the nurse prior to assignment. Safety and quality of care remain applicable during times of surge as do the need to match the level of competence with technologies and clinical interventions needed for the assignment; experience with the population served; and overall knowledge, skill and experience of the nurse with respect to the overall complexity of patient needs within an ICU setting.
- If a non-ICU nurse is re-deployed to a specialty area without experience within that area, it is the responsibility of the employer to provide just-in time training before deployment to the newly assigned area is confirmed to ensure the delivery of safe patient care.
- Making assignments that involve implementation of team-based care options that pair the nurse with an interprofessional team or utilization of acute care APRNs as team leads with RNs and ancillary staff is a solution to meet the needs of both the care team and the patient.
Upon re-deployment, request expectations and intervals of evaluating performance post assignment to identify areas needing support nurse and address any patient safety concerns.
If at any point you feel as though the conditions are unsafe, it is okay to speak up and say something. The safety of the nurse and staff AND the safety of the patient are equally important.
According to Provision 4 of ANA’s Code of Ethics for Nurses with Interpretive Statements, “The nurse has authority, accountability, and responsibility for nursing practice; makes decisions; and takes action consistent with the obligation to promote health and to provide optimal care.” Furthermore, within Provision 4.4 Assignment and Delegation of Nursing Activities or Tasks, “Nurses in management and administration have a particular responsibility to provide a safe environment that supports and facilitates appropriate assignment and delegation. This environment includes orientation and skill development; licensure, certification, continuing education, and competency verification; adequate and flexible staffing; and policies that protect both that patient and the nurse from inappropriate assignment or delegation of nursing responsibilities, activities, or tasks. Nurses in management or administration should facilitate open communication with health care personnel allowing them, without fear of reprisal, to express concerns or even to refuse an assignment for which they do not possess the requisite skill.”
Q: I am pregnant, what should I do?
A: A statement released by the Association of Women’s Health Obstetric and Neonatal Nursing (AWHONN) upholds “Normal physiologic and immunologic changes of pregnancy increase the pregnant woman’s susceptibility to infections. However, data specific to COVID-19 is limited. Pregnant women, their fetuses, and newborns may be at an increased risk for morbidity and mortality if COVID-19 is contracted during pregnancy.” In accordance with recommendations supported by AWHONN, ANA supports the CDC’s recommendation that health care facilities should consider limiting the exposure of pregnant health care personnel to patients with confirmed or suspected COVID-19 during high-risk procedures especially those with increased risk of aerosolization. If you are pregnant and are health care personnel, it is important to continue your routine prenatal care and inform your prenatal care clinician of your exposure risk at work along with any symptoms (including fever, cough, or difficulty breathing) associated with COVID-19. The Society for Maternal-Fetal Medicine (SMFM) and other pregnancy experts recommend that pregnant and lactating people be vaccinated against COVID-19. To read more about the studies confirming safety and efficacy of mRNA COVID-19 vaccines in pregnant and lactating persons, click here.
Q: I’m a breastfeeding mother and a nurse, am I safe at work?
A: Research on the risk of pregnant women and breastfeeding mothers associated with COVID-19 continues. The following guidelines are outlined by the CDC for breastfeeding mothers for the protection and prevention of spreading COVID-19 to their infants:
- Breast milk provides protection against many illnesses and is the best source of nutrition for most infants.
- You, along with your family and health care providers, should decide whether and how to start or continue breastfeeding.
- In limited studies, COVID-19 has not been detected in breast milk; however, we do not know for sure whether mothers with COVID-19 can spread the virus via breast milk.
- If you are sick and choose to direct breastfeed:
- Wear a facemask and wash your hands before each feeding.
- If you are sick and choose to express breast milk:
- Express breast milk to establish and maintain milk supply.
- A dedicated breast pump should be provided.
- Wash hands before touching any pump or bottle parts and before expressing breast milk.
- Follow recommendations for proper pump cleaning after each use, cleaning all parts that come into contact with breast milk.
- If possible, consider having someone who is well, feed the expressed breast milk to the infant.
For additional resources on breast feeding and pregnancy visit the CDC’s Guidance on Pregnancy and Breastfeeding.
Q: I work in a nursing home. What should I do?
A: Nursing homes have been severely impacted by COVID-19, with outbreaks causing high rates of infection, morbidity, and mortality. The vulnerable nature of the nursing home population combined with the inherent risks of congregate living in a healthcare setting, requires aggressive efforts to limit COVID-19 exposure and to prevent the spread of COVID-19 within nursing homes. The Centers for Medicaid and Medicare Services (CMS) has released guidelines and FAQs in preparation for reopening.
To aid in transparency of care for families and loved ones, effective May 8, 2020, all nursing homes must meet new COVID-19 reporting requirements mandated by CMS via an interim rule. These new guidelines require reporting of COVID-19 cases amongst staff and residents. Data will be gathered and monitored via the National Health and Safety Network. The COVID-19 Module will track:
- Resident Impact and Facility Capacity
- Staff and Personnel Impact
- Supplies and Personal Protective Equipment
- Ventilator Capacity and Supplies
Essential resources for the protection of your staff and residents can be found here:
- Toolkit on State Actions to Mitigate COVID-19 Prevalence in Nursing Homes, May 2020
- Association for Professionals in Infection Control and Epidemiology.
Watch the CDC’s mini infection control webinar series for frontline LTCF staff: These new webinars are a training tool/resource for frontline long-term care staff members.
Q: I work in home health or provide in home hospice care. What should I do?
A: Connect with your patient or patient’s family in advance by telephone, text monitoring system, or video conference prior to home visits for temperature and symptom monitoring. Document your assessment findings as a nursing note within the medical chart.
If you must visit the home and the patient is suspected or confirmed to have COVID-19, wear the following PPE: Gown, gloves, eye protection (goggles or face shield), N95 filtering facepiece or respirator (or medical facemask if not available). Always perform hand hygiene before donning PPE and after doffing PPE.
Don and doff PPE outside of the home, dispose of PPE outside the home in a lined trash receptacle, do not travel with used PPE.
The Centers for Medicare and Medicaid Services provides guidelines for home healthcare personnel along with detailed guidelines for home hospice care personnel.
Q: My employer purchased a supply of KN95 or N95 respirators from another country. What do I need to know?
A: As of June 30, 2021, the FDA announced it revoked the Emergency Use Authorizations for certain respirators and decontamination systems. To read the full statement, click here.
Find the FDAs appendix of authorized respirators here, and information from NIOSH on counterfeit respirators / misrepresentation of NIOSH-approval here.
Q: How can I evaluate if the gowns and gloves my facility provides will be adequate to protect me?
A: Information on how to assess the effectiveness of the different types of available gowns and gloves, and recommendations for the types of gowns and gloves to be used under various clinical situations, can be found on the CDC Personal Protective Equipment: Questions and Answers webpage for healthcare workers.
Q: Where can I find updated data and how can I plan for a patient surge?
A: COVID-19Surge - COVID-19Surge is a spreadsheet-based tool that hospital administrators and public health officials can use to estimate the surge in demand for hospital-based services during the COVID-19 pandemic.
COVID-NET - The Coronavirus Disease 2019-Associated Hospitalization Surveillance Network (COVID-NET) conducts population-based surveillance for laboratory-confirmed COVID-19-associated hospitalizations in children (persons younger than 18 years) and adults. Data presented on COVID-19-associated hospitalizations collected through COVID-NET are preliminary and subject to change as additional data are collected. Figures are based on varying denominators as selected variables may require more time to be collected. Data are refreshed and updated weekly. To learn more about the data, please visit: https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html
The Institute for Health Metrics and Evaluation (IHME) is an independent global health research center at the University of Washington.
The CDC Cases, Data, and Surveillance webpage allows you to track trends in Covid-19 testing, cases and deaths by County and State.
Ethical Considerations
Q: What is my obligation to care for patients versus keeping myself safe?
A: The Code of Ethics for Nurses states “the nurse’s primary commitment is to the patient”, and yet the nurse owes the same duty to self as to others. These equal obligations can conflict during pandemics when nurses must continually care for critically ill infectious patients, often under extreme circumstances including insufficient or inadequate resources and uncontained contagion. During pandemics, nurses and their colleagues must decide how much care they can provide to others while also taking care of themselves.
Q: I am concerned about the standard of care during the pandemic.
A: Professional nurses have a duty to care during crises like pandemics. Changes in the standard of care can occur in circumstances when available resources are limited or when a clinician is practicing in an unusual setting or with unfamiliar patient care needs. In a pandemic, nurses can find themselves operating in crisis standards of care environments. See ANA’s guidance for nurses in Crisis Standards of Care.
Q: We don’t have enough ventilators. What should I do?
A: An ethically sound framework should be used for health care organizations during public health emergencies for the fair allocation of resources, including ventilators and other lifesaving equipment. The Hastings Center has drafted guidelines to assist health care organizations when making these ethical decisions. The Hastings Center has drafted guidelines to assist health care organizations when making these ethical decisions.
Q: I am concerned about being retaliated against when I speak up about safety and other patient care issues.
A: ANA is disturbed about reports of employers retaliating against nurses and other health care workers for raising legitimate concerns about their personal safety while caring for patients with COVID-19. Reports of intimidation, firing, ostracizing, and more are unacceptable. Nurses who are experiencing acts of retaliation from their employer are urged to file a whistleblower complaint online with Occupational Safety and Health Administration (OSHA) or call 1-800-321-OSHA (6742). For more information please see ANA’s news release on this issue.
Mental Health and Well-being
Q: I am completely overwhelmed. What can I do to help myself?
A: Coping mentally and emotionally with the COVID-19 pandemic is difficult for everyone, but even more so for nurses. Please know that you are not alone-you are providing care to a grateful nation, and the American Nurses Association honors your service.
Below, you will find two categories of resources to assist nurses and other health care providers with mental health resources. The first category is for dealing with stress, fear, and anxiety associated with the COVID-19 Pandemic. You may also want to check with your employer, university, state, specialty nurse association, or your state board of nursing about their resources including any employee assistance program (EAP) or peer assistance/counseling programs. The second category contains national lifelines and helplines.
If your stress, anxiety, or fear causes you to think about suicide or be in crisis, call the National Suicide Prevention Lifeline at 1-800-273-TALK.
Resources
- American Nurses Foundation Well-Being Initiative
- ANA Nurse Suicide Prevention/Resilience webpage
- ANA Healthy Nurse, Healthy Nation
- ANA Healthy Nurse Healthy Nation blog: Mental Health Help for Nurses
- American Psychiatric Nurses Association Managing Stress & Self Care during COVID-19: Information for Nurses
- Six Tips for Nurses Coping with the COVID-19 Pandemic A blog by Dr. Bernadette Melnyk and ANA’s Healthy Nurse, Healthy Nation™
- Substance Abuse and Mental Health Services Administration (SAMHSA) Suicide Prevention Resource Center (SPRC) Resources to Support Mental Health and Coping with the Coronavirus (COVID-19)
- Coronavirus and Mental Health: Taking Care of Ourselves during Infectious Disease Outbreaks A blog from the American Psychiatric Association, includes printable hand out
- Uniformed Services University’s Center for the Study of Traumatic Stress’ Sustaining the Well-being of Healthcare Personnel during Coronavirus and Other Infectious Outbreaks
- American Holistic Nurses Association’s Holistic Stress Management website
- National Alliance on Mental Illness (NAMI) Navigating a Mental Health Crisis
- NAMI’s Navigating a Mental Health Crisis Infographic
- US Department of Veterans Affairs (VA) National Center for PTSD’s Managing Healthcare Workers’ Stress Associated with the COVID-19 Virus Outbreak
- SAMHSA Suicide Prevention Resource Center website
National Life-and Helplines
- National Suicide Prevention Lifeline 1-800-273-TALK (8255)
- SAMHSA National Helpline 1-800-662 HELP (4357)
- SAMHSA Disaster Distress Helpline 1-800-985-5990
Licensure and Credentialing guidelines
Q: What does an emergency declaration mean with regard to my nursing license?
A: This varies greatly by state and is evolving. Some general themes include granting an extension for license renewal, the ability for nurses to cross state borders to practice, waiving of restrictions imposed on advanced practice registered nurses (APRNs) and exemptions for inactive/retired nurses.
A comprehensive source to determine how you may be impacted can be found on The National Council of State Boards of Nursing’s (NCSBN) website. The site is updated daily, Monday through Friday.
Additional information on licensure and credentialing can be found on the ANA Covid-19 Licensure and Credentialing Guidelines webpage.
Legislative and Regulatory
Q: How do I set up a telehealth program for my patients?
A: The practice of telehealth will provide a continuation of health services while protecting providers and patients from exposure to COVID-19. To successfully implement a telehealth program, it is essential to understand laws and regulations associated with privacy protection and virtual care. Learn more about starting a telehealth program through the American Association of Nurse Practitioners (AANP) Telehealth Updates.
Other useful resources include The U.S. Department of Health and Human Services FAQs on Telehealth and HIPAA during the COVID-19 nationwide public health emergency.
Q: What is ANA doing to get more PPE to the frontlines?
A: ANA is engaged in ongoing advocacy efforts to support all health care personnel responding to the COVID-19 pandemic. We continue aggressively pushing Congress and the Administration to take action to ramp up the production of PPE. Through our RN Action campaign, nurses and the general public can write a letter that is submitted to Congress asking for more PPE on the frontlines. We have remained present throughout media outlets such as CNN, MSNBC, and NPR, and on social media through Twitter Chats, polls, and posts working to keep this issue at the forefront. Below is a list of letters written to date advocating for PPE, medical equipment, increase in testing capacity along with our statement condoning retaliation against nurses within the workplace.
- April 9, 2020 - ANA Disturbed by Reports of Retaliation Against Nurses for Raising Concerns About COVID-19 Safety
- April 8, 2020 - ANA Letter to President Trump
- April 2, 2020 - ANA Letter to HHS Secy Azar Regarding Special Enrollment for Federal Health Insurance Exchange
- March 24, 2020 - Joint AHA/AMA/ANA Letter to the American Public Regarding Coronavirus
- March 21, 2020 - Joint AHA/AMA/ANA Letter to the President Requesting Immediate Use of the Defense Production Act
- March 19, 2020 - Joint AHA/AMA/ANA Letter to Capitol Hill seeking $100 billion for frontline health care workers
- March 18, 2020 Joint Industry Letter to Administration & Capitol HIll recommending actions to address COVID-19
- March 16, 2020 Joint AHA/AMA/ANA Letter to Capitol Hill seeking $1B for comprehensive strategy in response to COVID-19
- March 12, 2020 ANA Mask Transparency Letter to Leadership
- March 12, 2020 Joint AHA/AMA/ANA Letter to Vice President Michael Pence
- March 11, 2020 ANA Letter to Honorable Michael Pence in response to the work of the Administration and the Coronavirus Task Force
Q: I am concerned about being retaliated against when I speak up about safety and other patient care issues.
A: ANA is disturbed about reports of employers retaliating against nurses and other health care workers for raising legitimate concerns about their personal safety while caring for patients with COVID-19. Reports of intimidation, firing, ostracizing, and more are unacceptable. Nurses who are experiencing acts of retaliation from their employer are urged to file a whistleblower complaint online with Occupational Safety and Health Administration (OSHA) or call 1-800-321-OSHA (6742). For more information please see ANA’s news release on this issue.
Consumers (what to tell consumers/your patients)
Q: How should I protect myself from this virus?
A: An updated vaccination is the best defense against SARS CcV-2 and associated COVID-19. Everyday measures to protect yourself from the virus include frequent hand hygiene, improving indoor ventilation, spending more time outdoors, avoiding exposure to those with COVID-19, and wearing a face mask in areas of high transmission. When making decisions about prevention strategies and behaviors, you should consider the COVID-19 hospitalization level in the area where you live, work, or visit, and make decisions based on whether you or a close contact are at high risk for severe illness. If you are wearing a mask, experts recommend you upgrade your mask to a high filtration respirator if you want optimal protection. If you think you have been exposed to Covid-19, remember to test, and treat early! If you test positive for COVID-19 and have one or more health conditions that increase your risk of becoming very sick, treatment may be available. Contact a health professional right away after a positive test to determine if you may be eligible, even if your symptoms are mild right now. Depending on where you live, there may be a COVID-19 Test-To-Treat location near you. Don’t delay, treatment must be started within the first few days to be effective.
See also the CDC How to Protect Yourself and Others webpage.
Q: I am COVID-19 positive, but not sick enough to be admitted to the hospital. What should I do to take care of myself at home ?
A: If you are COVID-19 positive, the CDC provides the following guidance to help prevent COVID-19 from spreading between people in homes and communities:
Stay home for at least 5 days
• Stay home for 5 days and isolate from others in your home. Most people with COVID-19 have mild illness and can recover at home without medical care. Do not leave your home, except to get medical care. Do not visit public areas and do not go to places where you are unable to wear a mask
• Take care of yourself. Get rest and stay hydrated. Take over-the-counter medicines, such as acetaminophen, to help you feel better.
• Stay in touch with your doctor. Call before you get medical care. Be sure to get care if you have trouble breathing, or have any other emergency warning signs, or if you think it is an emergency.
• Do not travel and avoid public transportation, ride-sharing, or taxis if possible.
Separate yourself from other people
As much as possible, stay in a specific room and away from other people and pets in your home. Increase ventilation in the room by opening a window or using a Hepa filter. If possible, you should use a separate bathroom. If you need to be around other people or animals in or outside of the home, wear a well-fitting mask.
Tell your close contacts that they may have been exposed to COVID-19
An infected person can spread COVID-19 starting 48 hours (or 2 days) before the person has any symptoms or tests positive. By letting your close contacts know they may have been exposed to COVID-19, you are helping to protect everyone.
Monitor your symptoms
• Symptoms of COVID-19 include fever, cough, or other symptoms.
• Follow care instructions from your healthcare provider and local health department. Your local health authorities may give instructions on checking your symptoms and reporting information.
When to seek emergency medical attention
• Trouble breathing, persistent pain or pressure in the chest, new confusion, inability to wake or stay awake, pale, gray, or blue-colored skin, lips, or nail beds, depending on skin tone. This list is not all possible symptoms. Please call your medical provider for any other symptoms that are severe or concerning to you.
• Call 911 or call ahead to your local emergency facility: Notify the operator that you are seeking care for someone who has or may have COVID-19.
Call ahead before visiting your doctor
• Call ahead. Many medical visits for routine care are being postponed or done by phone or telemedicine.
• If you have a medical appointment that cannot be postponed, call your doctor’s office, and tell them you have or may have COVID-19. This will help the office protect themselves and other patients.
If you are sick, wear a well-fitting mask
• You should wear a mask if you must be around other people or animals, including pets (even at home).
• Wear a mask with the best fit, protection, and comfort for you.
• You don’t need to wear the mask if you are alone. If you can’t put on a mask (because of trouble breathing, for example), cover your coughs and sneezes in some other way. Try to stay at least 6 feet away from other people. This will help protect the people around you.
• Masks should not be placed on young children under age 2 years, anyone who has trouble breathing, or anyone who is not able to remove the mask without help.
Cover your coughs and sneezes
• Cover your mouth and nose with a tissue when you cough or sneeze, and throw away used tissues in a lined trash can.
• Immediately wash your hands with soap and water for at least 20 seconds. If soap and water are not available, clean your hands with an alcohol-based hand sanitizer that contains at least 60% alcohol.
Clean your hands often
• Wash your hands often with soap and water for at least 20 seconds. This is especially important after blowing your nose, coughing, or sneezing; going to the bathroom; and before eating or preparing food.
• Use hand sanitizer if soap and water are not available. Use an alcohol-based hand sanitizer with at least 60% alcohol, covering all surfaces of your hands and rubbing them together until they feel dry.
• Soap and water are the best option, especially if hands are visibly dirty.
• Avoid touching your eyes, nose, and mouth with unwashed hands.
• Handwashing Tips
Avoid sharing personal household items
• Do not share dishes, drinking glasses, cups, eating utensils, towels, or bedding with other people in your home.
• Wash these items thoroughly after using them with soap and water or put in the dishwasher.
Clean surfaces in your home regularly
• Clean and disinfect high-touch surfaces (for example, doorknobs, tables, handles, light switches, and countertops) in your “sick room” and bathroom. In shared spaces, you should clean and disinfect surfaces and items after each use by the person who is ill.
• Clean visible dirty surfaces with household cleaners containing soap or detergent. Then, use a household disinfectant. Use a product from EPA’s List N: Disinfectants for Coronavirus (COVID-19), and follow the instructions on the label to ensure safe and effective use of the product. You may also need to wear personal protective equipment, such as gloves, depending on the directions on the product label. Immediately after disinfecting, wash your hands with soap and water for 20 seconds. Find complete guidance on cleaning, disinfecting and ventilation here.
Take steps to improve ventilation at home
• Improve ventilation (air flow) at home to help prevent from spreading COVID-19 to other people in your household.
• Clear out COVID-19 virus particles in the air by opening windows, using air filters, and turning on fans in your home.
• Use this interactive tool to learn how to improve air flow in your home.
Deciding when you can be around others after being sick with COVID-19 is different for different situations.
Q: Information for the general public about the use of cloth masks?
A: Fully vaccinated individuals can go without a mask in some settings if permitted to do so by their state, locale, or territory. However, it is recommended that you continue to wear your mask even if vaccinated in areas with high transmission. A mask will maximize your protection and prevent spreading it to others. When making decisions about individual prevention strategies and behaviors, you should consider the COVID-19 hospitalization levels in the area where you live, work, or visit, and make decisions based on whether you, or a close contact are at high risk for severe illness. If you are wearing a mask, experts recommend you upgrade your mask to a high filtration respirator if you want optimal protection.
Regardless of the level of transmission in your area, if you have a weakened immune system, are at increased risk for severe disease, wearing a mask provides additional protection. You must also continue to wear a mask where required by laws, rules, regulations, or local guidance.
Additional information on the Use of Masks to Slow the Spread of COVID-19, can be found on the CDC’s website.
Give to the Coronavirus Response Fund for Nurses (CRFN)
Q: I want to donate. Where should I go?
A: The Coronavirus Response Fund for Nurses supported by the American Nurses Foundation Nurses is another way to help support nurses on the frontlines of the COVID-19 response. We have created a Coronavirus Response Fund for Nurses to enable the public to support and thank nurses. The national fund will address the identified, emerging needs of nurses and will focus on:
- Providing direct assistance to nurses
- Supporting the mental health of nurses – today and in the future
- Ensuring nurses everywhere have access to the latest science-based information to protect themselves, prevent infection, and care for those in nee
- Driving the national advocacy focused on nurses and patients
To share your thanks and support for nurses on the frontlines you can make an online donation to the American Nurses Foundation Coronavirus Response Fund for Nurses.
You can also Text THANKS to 20222 to make a $10 donation. The $10 donation will be added to your mobile phone bill and remitted to the American Nurses Foundation Coronavirus Response Fund for Nurses.
Q: I want to learn about the good work that the Response Fund has done so far. Where can I learn more about that?
A: Based on the needs of American Nurses, the American Nurses Foundation developed the Coronavirus Response Fund for Nurses with 4 distinct programmatic pillars in mind. Foundation staff have developed the following pages as our work advances and the pillars take shape. Please use the following links to learn more about the work being done by the Response Fund:
- Mental Health & The Well-Being Initiative
- Advocating for Nurses and Those They Serve
- The Latest Science-based Information
- Direct Financial Assistance
Q: I want to learn about the Response Fund donors, who they are and what they have done to raise money for American Nurses. Where can I learn more about that?
A: Support for the Response Fund comes from a broad range of both individuals and corporations. We wanted to honor their generosity and fundraising resourcefulness/creativity by creating a dedicated space to highlight their work. Learn more about the donors and their fundraising methods on the following pages:
Volunteer
Q: I am a retired nurse. How can I help?
A: First, you need to determine if your state or the state in which you are planning to work has provisions specific to retired nurse’s ability to re-enter the workforce and if those have been relaxed during the emergency response. It is also critical to self-assess your current competence based on anticipated contributions and identify if there is support for filling any gaps. You can find additional volunteer information here.
Q: I want to volunteer. What should I know?
A:
- If you are nurse or medical personnel seeking to volunteer in response to the COVID-19 pandemic, here is a list of resources where you can volunteer your time and service:
- Medical Reserve Corps
- Locate your state’s public health emergency coordinator
- American Red Cross
- Policy Brief: U.S. Nursing Leadership Supports Practice/Academic Partnerships to Assist the Nursing Workforce during the COVID-19 Crisis
- Designated Health
- Follow your local and/or state directives on sheltering in place and maintaining social distance. It is important that we do everything to slow and stop the spread of COVID-19 to reduce the current and future strain on the health care system.
- Reach out to the volunteer service department or chaplain at your local hospital and ask what type of assistance is needed.
- Don’t forget about reaching out to the local emergency response agencies. They too are feeling the pressure of this pandemic.
- If you are well, consider making an appointment to donate blood at a Red Cross or other blood services donation center.
- Check your local media services websites. They often post information on ways to help.
- If you are interested in donating items or services reach out to the state or local public and/or emergency response coordinators.
It is also important to consider other factors that may affect your ability to volunteer.


